In the chemical industry, optimizing reactions is essential to improve efficiency, reduce costs, minimize waste, and ensure the quality of products. Chemical reactions are complex processes influenced by various factors, each of which must be carefully controlled to achieve the desired outcome. This article will break down the key factors involved in optimizing chemical reactions in industrial production, providing practical strategies to ensure successful large-scale operations.
1. Reaction Temperature
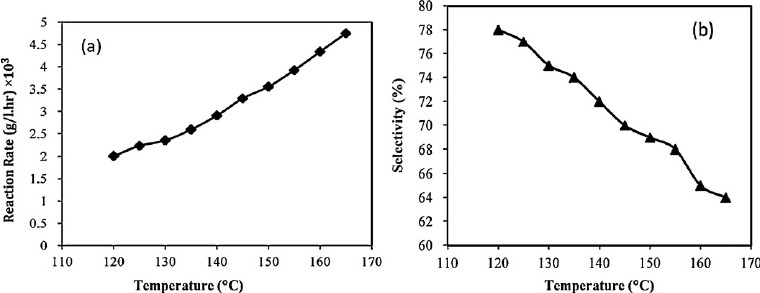
Temperature is a critical factor that directly impacts reaction rates and product yield. According to the Arrhenius equation, reaction rates typically increase exponentially with temperature, as higher temperatures provide more energy to overcome the activation barrier of the reaction. However, excessive heat can lead to undesirable side reactions, degradation of sensitive compounds, or the formation of by-products.
Strategies for Temperature Optimization:
- Monitoring Reaction Kinetics: Determining the optimal temperature range for maximum reaction rate without compromising product quality is crucial. Reaction kinetics studies, typically done at different temperatures, help identify this balance.
- Use of Temperature Control Systems: In large-scale chemical production, precise temperature control systems, such as jacketed reactors or heat exchangers, are essential. These systems can maintain a constant temperature and allow for gradual heating or cooling to prevent thermal degradation.
- Exothermic Reactions: For exothermic reactions, managing heat removal is vital. Overheating can cause runaway reactions, leading to safety risks and product loss. Installing adequate cooling systems, like circulating chilled water or cryogenic fluids, can help mitigate this risk.
2. Pressure Control
Pressure plays a significant role, especially in gas-phase reactions or those involving volatile components. According to Le Chatelier’s principle, increasing pressure often shifts the equilibrium toward the side with fewer moles of gas, enhancing the yield of certain reactions. In reactions involving gases, pressure can also affect reaction kinetics.
Strategies for Pressure Optimization:
Pressure Reactors: Utilizing high-pressure reactors or autoclaves can facilitate reactions that require elevated pressures, such as hydrogenation or polymerization processes.
Gas Flow Control: For reactions involving gaseous reactants or products, maintaining a steady flow rate ensures that the reactants are constantly supplied at the right pressure. Gas mass flow controllers and pressure regulators are commonly used in these setups.
Avoiding Over-pressurization: While higher pressure can improve yields in some cases, exceeding optimal levels may pose safety risks or lead to equipment damage. Safety valves and pressure relief systems are necessary to prevent over-pressurization.
3. Catalyst Use and Selection
Catalysts are substances that speed up chemical reactions without being consumed. The selection and optimization of a catalyst are key to improving reaction rates and selectivity, particularly in industrial processes like refining, polymer production, and pharmaceutical synthesis. An ideal catalyst increases reaction speed while minimizing unwanted side reactions.
Strategies for Catalyst Optimization:
Catalyst Surface Area: For heterogeneous catalysts (solid catalysts interacting with gaseous or liquid reactants), the surface area is critical. Increasing the surface area, through techniques like catalyst impregnation on porous supports, improves the number of active sites available for the reaction.
Catalyst Regeneration: Over time, catalysts can become deactivated due to fouling, coking, or poisoning. Regular regeneration of the catalyst, such as by removing impurities or burning off carbon deposits, extends its life and maintains efficiency.
Homogeneous vs. Heterogeneous Catalysis: Choosing between homogeneous (catalyst in the same phase as reactants) and heterogeneous catalysis depends on the nature of the reaction. Homogeneous catalysis often provides better selectivity but can be harder to separate from the product, while heterogeneous catalysts are easier to recover and reuse.
4. Reaction Time
The duration of a reaction impacts both yield and selectivity. Reactions need sufficient time to reach completion, but prolonged reaction times can lead to the formation of by-products or degradation of sensitive compounds. Understanding reaction kinetics is essential for determining the optimal reaction time.
Strategies for Reaction Time Optimization:
Batch vs. Continuous Reactions: In batch reactors, the reaction time is controlled by stopping the process once the desired conversion is achieved. However, in continuous reactors, such as plug flow or stirred-tank reactors, optimizing residence time (the time reactants spend in the reactor) ensures consistent product quality.
Monitoring Reaction Progress: Techniques like in-line spectroscopy, chromatography, or mass spectrometry allow real-time monitoring of reactants and products. This enables timely adjustments to reaction conditions, ensuring the reaction is stopped at the optimal point.
Scaling Up Reaction Time: When scaling up from lab to industrial scale, maintaining the same reaction time can be challenging due to differences in heat and mass transfer. Reactors with efficient mixing systems, such as agitators or baffles, help ensure uniformity and reduce time discrepancies.
5. Concentration of Reactants
Reactant concentration is another important factor influencing the rate of reaction. According to the law of mass action, increasing the concentration of reactants generally increases the reaction rate. However, too high a concentration can lead to issues such as equipment fouling or the formation of side products.
Strategies for Concentration Optimization:
Optimal Stoichiometry: Using the correct stoichiometric ratio of reactants ensures that the reaction proceeds efficiently without excess reactant waste. In industrial production, small deviations from stoichiometry can result in significant material and cost inefficiencies.
Feed Systems for Continuous Reactors: In continuous processes, maintaining a steady feed of reactants at the optimal concentration is crucial. Automated feed systems, controlled by feedback loops, allow for precise control of reactant concentrations and avoid fluctuations that can affect reaction performance.
Dilution and Solvent Effects: In some reactions, using solvents to dilute reactants can prevent side reactions or control reaction heat. However, solvent choice must be optimized to avoid negative effects on the reaction rate or product yield.
6. Mixing and Mass Transfer
Efficient mixing ensures that reactants come into contact with each other and with catalysts. Poor mixing can result in uneven reaction conditions, hot spots, or incomplete reactions. In large reactors, mass transfer limitations (the movement of reactants to the reaction sites) can slow down the overall reaction rate.
Strategies for Mixing and Mass Transfer Optimization:
Agitators and Mixing Systems: In stirred-tank reactors, agitators or impellers improve mixing and ensure uniform distribution of heat and reactants. Choosing the right type and speed of agitator is crucial for achieving optimal results.
Baffles in Reactors: Installing baffles (vertical plates) inside reactors prevents vortex formation and enhances mixing. This is particularly important in large-scale reactors where poor mixing can lead to inefficiencies.
Improving Mass Transfer: In heterogeneous reactions, mass transfer between phases (such as between a gas and liquid) can be a limiting factor. Using high-surface-area catalysts or increasing gas-liquid contact, such as through bubbling systems, improves mass transfer rates.
Optimizing chemical reactions in industrial production involves careful control of temperature, pressure, catalyst selection, reaction time, reactant concentration, and mixing. Each factor plays a significant role in determining reaction efficiency, yield, and safety. By systematically analyzing and adjusting these parameters, industries can improve their chemical processes, leading to more efficient, cost-effective, and sustainable production.
Applying these strategies not only enhances production efficiency but also contributes to greener, more sustainable manufacturing processes, aligning with the growing demand for environmentally responsible industrial practices.